Project Overview
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality worldwide,
responsible for approximately 17.9 to 20.5 million deaths annually. Whilst the global burden of CVD keeps increasing, recent efforts to accelerate the development of innovative therapeutic strategies for managing CVD have persistently failed to deliver new drugs to the market. These challenges underscore significant barriers to successfully translating benchside research into effective clinical applications for CVD management. By retaining similar proliferative capabilities, self-organizing properties, and contractility as the heart tissue, laboratory networks of cardiac cells offer an ideal platform to map the process of cardiac tissue formation, dynamical interaction and deterioration under physiological and pathological conditions. By developing novel ML systems integrating computer vision and image segmentation strategies, this project aims to automate and refine the extraction of quantitative and time-resolved data on individual cardiac cells’ and cardiac cell networks’ morphological organization, contractility, spatiotemporal signalling, and cell-to-cell synchronisation. This approach will offer novel insights into the early mechanisms underpinning the development of CVD in humans.
Period: October 2024 – October 2027
Impact: This research will develop advanced ML tools to analyse cardiac cell images and videos, creating a valuable resource for cardiac bioimaging. These frameworks will provide novel insights into the spatial and temporal dynamics of cardiac network behaviour. The outcomes of this work will contribute to a deeper understanding of the mechanisms driving the functional decline of the heart muscle, ultimately supporting more accurate diagnoses and improved therapies for CVD.
Project Aims
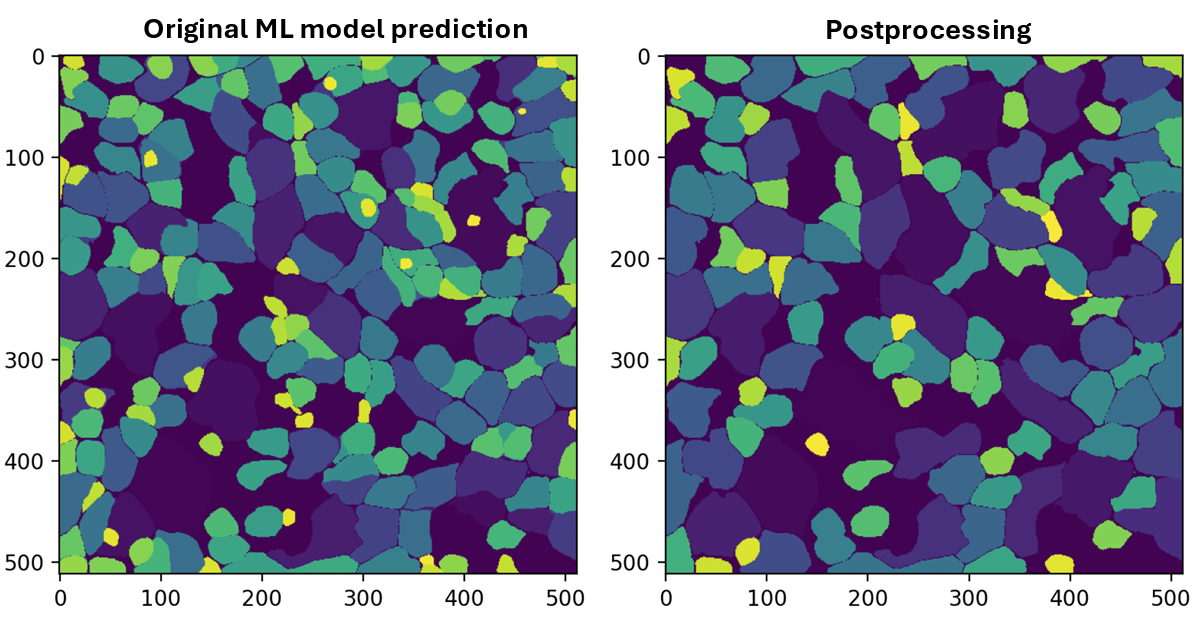
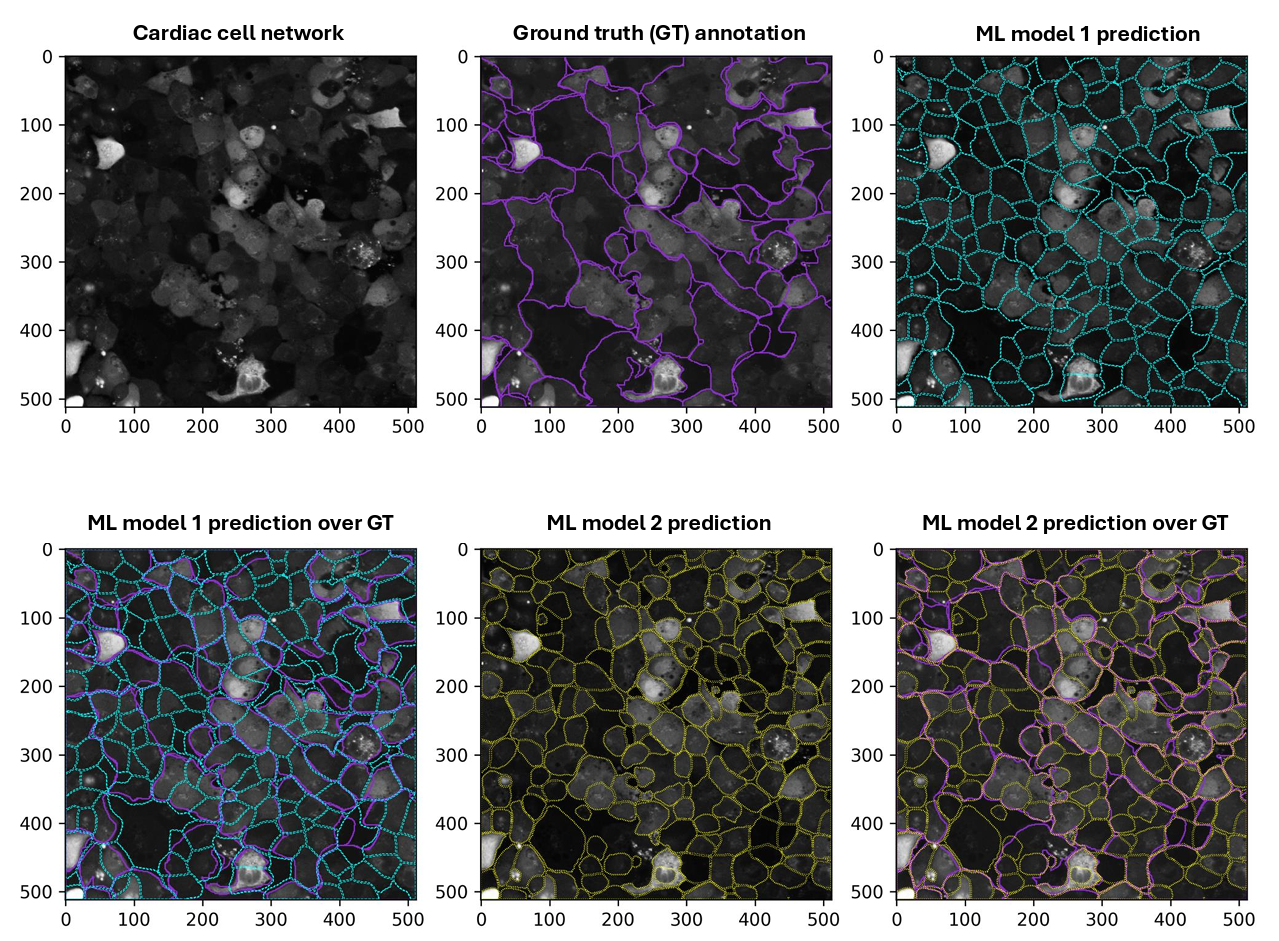
• Develop ML algorithms for the segmentation and dynamic analysis of images and live recordings of laboratory cardiac cell networks.
• Automate the extraction of quantitative data on the morphological organization, contractility, spatiotemporal signalling, and synchronisation of functionally and structurally coupled cardiac cells organised into complex network architectures.
• Improve the understanding of early events underpinning the functional decline of heart muscle in CVD to inform better diagnosis and therapeutic interventions.
Project Team
Collaborators
Swansea University, UK Research and Innovation (UKRI), Morgan Advanced Studies Institute (MASI)